- 1School of Health Management, Xihua University, Chengdu, China
- 2Faculty of Geosciences and Environmental Engineering, Southwest Jiaotong University, Chengdu, China
- 3School of Food and Biological Engineering, Xihua University, Chengdu, China
- 4Health Promotion Center, Xihua University, Chengdu, China
In the coronavirus disease 2019 (COVID-19) pandemic, vaccinations were essential in preventing COVID-19 infections and related mortality in older adults. The objectives of this study were to evaluate the effectiveness and safety of the COVID-19 vaccines in older adults. We systematically searched the electronic bibliographic databases of PubMed, Web of Science, Embase, Cochrane Library, ClinicalTrials.gov, Research Square, and OpenGrey, as well as other sources of gray literature, for studies published between January 1, 2020, and October 1, 2022. We retrieved 22 randomized controlled trials (RCTs), with a total of 3,404,696 older adults (aged over 60 years) participating, that were included in the meta-analysis. No significant publication bias was found. In the cumulative meta-analysis, we found that the COVID-19 vaccines were effective in preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (OR = 0.38, 95% CI = 0.23–0.65, p = 0.0004) and in reducing the number of COVID-19-related deaths (OR = 0.16, 95% CI = 0.10–0.25, p < 0.00001) in elderly people. Antibody seroconversion (AS) and geometric mean titer (GMT) levels significantly increased in vaccinated older adults [OR = 24.42, 95% CI = 19.29–30.92; standardized mean difference (SMD) = 0.92, 95% CI = 0.64–1.20, respectively]. However, local and systemic adverse events after COVID-19 vaccine administration were found in older adults (OR = 2.57, 95% CI = 1.83–3.62, p < 0.00001). Although vaccination might induce certain adverse reactions in the elderly population, the available evidence showed that the COVID-19 vaccines are effective and tolerated, as shown by the decrease in COVID-19-related deaths in older adults. It needs to be made abundantly clear to elderly people that the advantages of vaccination far outweigh any potential risks. Therefore, COVID-19 vaccination should be considered as the recommended strategy for the control of this disease by preventing SARS-CoV-2 infection and related deaths in older adults. More RCTs are needed to increase the certainty of the evidence and to verify our conclusions.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022319698, identifier CRD42022319698.
Introduction
The emergence and spread of coronavirus disease 2019 (COVID-19) brought about negative effects and unprecedented challenges that affected the physical and mental well-being of people worldwide (1). According to a World Health Organization (WHO) report, as of October 1, 2022, there have been more than 616.95 million cumulative cases of COVID-19 globally, including more than 6.5 million deaths (2). A meta-analysis showed that mortality increases from 9.5% in patients 60–69 years old up to 29.6% in those aged >80 years (3). In another study, adults aged 65 years and older were found to be 8.7 times more likely to require hospitalization for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and have accounted for 22% of cases and up to 78% of COVID-19-related deaths (4). Due to the lower efficacy of treatment for severe COVID-19, older adults have poorer clinical outcomes, including a greater chance of 30-day hospitalization and mechanical ventilation, which also potentially leads to higher mortality rates (5).
As a result, there was a critical need to focus on the vaccination of older adults against SARS-CoV-2 infection and to lower their risk of severe disease and mortality. Although various vaccines and treatments have continuously emerged, these have remained unable to completely control the spread of the virus and eliminate infections. After COVID-19 infection, bodily injury becomes very serious, especially in elderly people (6–11). In the absence of definitive treatment, the development of vaccines against COVID-19 was perceived as an effective strategy to contain the spread of the pandemic.
As of October 1, 2022, there were 177 COVID-19 vaccines in clinical development, 199 in preclinical development, and 11 in phase 4 clinical trials (12). At this time, the WHO had approved 11 COVID-19 vaccines for emergency use listing (EUL), which are shown in Appendix 1 (13). More and more vaccines are now being approved for marketing and undergoing evaluation by the WHO EUL/PQ. The status of each of the 44 COVID-19 vaccines within the WHO EUL/PQ evaluation process is shown in Appendix 2 (14).
COVID-19 vaccines have become more readily available all around the world. Timely vaccination and high vaccination rates are necessary to effectively control diseases (15). Thus far, a total of 163.2 billion vaccine doses have been administered and 28 people per 100 population boosted worldwide, with China having the highest cumulative number of vaccine doses, followed by the United States (2).
Age is an important factor affecting the spread of and infection with COVID-19. Compared with young people, elderly people are more likely to be infected with COVID-19 and more likely to experience serious illness after infection, and their hospitalization rates and mortality rates after infection are higher than those of young people (8–10). Since older adults have the highest rates of COVID-19 mortality, many countries have invested more resources into finding better strategies to achieve and sustain higher vaccination coverage in the older adult population (16). However, there is still disbelief and hesitation about the effectiveness of COVID-19 vaccines. Vaccine hesitancy and rejection (VHR) is one of the top 10 threats to global health (17, 18). The effectiveness and safety of the COVID-19 vaccines against COVID-19 infection therefore need to be assessed in older people.
Clinical trials have shown COVID-19 vaccines to be immunogenic against SARS-CoV-2 infection and safe, with their efficacy ranging from 86% and 95% for the messenger RNA (mRNA) vaccines BNT162b2 (19) and mRNA-1273 (20), respectively, to 74% for the AZD1222 (ChAdOx1 nCoV-19) vaccine (21) in those aged 18 years and older. The other vaccines included in this review have been found to have intermediate efficacies of 67% for Ad26.COV2.S (22) and 78% for the inactivated SARS-CoV-2 vaccine (23) in adults (aged ≥18 years). Furthermore, the COVID-19 vaccines have also been shown to be immunogenic against SARS-CoV-2 infection and safe in older adults (aged ≥60 years), a result similar to that seen in young and middle-aged people (aged from 18 to 60 years) (19–23). In some randomized controlled trials (RCTs), all vaccine formulations have been well tolerated overall, and vaccine-related adverse events or outcomes after vaccination have been generally mild to moderate and transient in adults (19–24).
Although the current COVID-19 vaccination guidelines for older people differ by country, the WHO recommends vaccination with the mRNA, recombinant adenovirus vector, or inactivated coronavirus vaccines, among others, for all older people (25). Older people, as a specific population, are at very high risk of adverse outcomes from infectious diseases due to comorbidities associated with aging and their decreased immunological competence (immunosenescence) (26). Immunosenescence not only increases susceptibility to SARS-CoV-2 infection but also limits the effectiveness of the COVID-19 vaccines, which may lead to differences in vaccine effectiveness between younger (<55 years old) and older people. Vaccine formulations effective in younger people might not engender immunity in older populations (27). Furthermore, there have been concerns that the currently available COVID-19 vaccines may not be adequate to protect older people from COVID-19 infection. A number of studies have revealed that older people had a higher rate of vaccination hesitancy and distrust compared to the general population owing to uncertainties and fears associated with vaccine side effects (28–30). Consequently, it is vital to conduct a meta-analysis on the efficacy and safety of the COVID-19 vaccines in older people, which would provide additional scientific data that could be helpful in protecting older people, a vulnerable demographic during the COVID-19 pandemic.
Therefore, in this meta-analysis and systematic review, we aimed to summarize the overall effectiveness and safety of the COVID-19 vaccines against COVID-19 infection in older people in order to provide evidence for an improved vaccine strategy for this population.
Methods
Data sources and search strategy
PubMed, Web of Science, Embase, Cochrane Library, ClinicalTrials.gov, Research Square, and open gray and gray literature were searched in the Chinese and English languages from January 1, 2020, to October 1, 2022. In addition, we also manually searched for articles that met the criteria. The search mesh terms included (“older adults” OR “old people” OR “old population” OR “the aged” OR “elder people” OR “the elderly” OR “older patients” OR “aging” OR “gerontology”) AND (“COVID-19” OR “coronavirus” OR “SARS-CoV-2” OR “variant strain” OR “Delta variant” OR “B.1.617.2” OR “Omicron variant” OR “B.1.1.529”) AND (“vaccine” OR “vaccination”) AND (“randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “randomly” OR “trial”), as shown in Appendix 3. Zotero 6.0.4 (https://www.zotero.org/) was used to manage and screen records and to exclude duplicates. This meta-analysis and systematic review were performed in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; checklist provided in Appendix 4). This study was registered on PROSPERO (CRD42022319698).
Data selection criteria
This study included RCTs that evaluated the effectiveness and safety of COVID-19 vaccines in older adults (aged ≥60 years). Studies that met the following criteria were excluded: a) irrelevant to the subject of the meta-analysis (SARS-CoV-2 vaccination not involved); b) reviews, editorials, conference papers, case reports, or animal experiments; c) duplicate studies or studies with overlapping participants; d) unable to confirm diagnosis of COVID-19; and e) those with insufficient data to calculate the outcomes in terms of the effectiveness and safety of SARS-CoV-2 vaccines.
Data screening and extraction
The references of the retrieved studies were screened to further select relevant studies suitable for inclusion in this meta-analysis. Data extraction was performed by two independent investigators based on the inclusion and exclusion criteria. The collected documents were processed as references using the document management software Zotero. Any disagreements were discussed with a third investigator. The following materials were extracted from each article by two independent investigators: a) basic information on the studies, including the first author, publication year, and study design; b) characteristics of the study population, including sample sizes, age groups, gender groups, and setting or locations; c) types of SARS-CoV-2 vaccines and the number of doses administered; d) outcomes in terms of the effectiveness of the SARS-CoV-2 vaccines, including the following: number of laboratory-confirmed COVID-19 infections, hospitalizations for COVID-19, admissions to the intensive care unit (ICU) for COVID-19, COVID-19-related deaths, number and sample origin of antibody titer or seroconversion rates, and number of interferon gamma (IFN-γ)-positive T cells; and e) outcomes in terms of the safety of the SARS-CoV-2 vaccines.
Risk of bias and quality assessment
The Cochrane collaboration risk-of-bias tool RoB 2 IRPG beta v9 was used to assess all potential sources of bias in the included references (31), while the GRADE system was used to assess the quality of evidence for all systematic reviews (32, 33).
The Cochrane evaluation criteria include the following five aspects (31): randomization process, deviation from the intended intervention, missing outcome data, measurement of the outcome, and selection of reported results. Publication bias was visualized using funnel plots. Two reviewers (KX and ZW) independently assessed the risk of bias during the evaluation process. Any disagreements were resolved by negotiation or with the participation of a third reviewer (XM or JW). In accordance with the Cochrane Statement of Risk of Bias, the risk of bias for each study was rated as high, some concern, or low risk. Studies with a high overall risk of bias for any single outcome were excluded from the meta-analysis.
The quality of evidence according to the GRADE system was evaluated based on the following five aspects (32, 33): study design limitations, consistency between studies, directness (ability to generalize), precision of results (sufficient or precise data), and publication bias. In accordance with the scoring criteria of the GRADE system, the quality of evidence was classified into five levels: high, moderate, low, very low quality, or no evidence. Similarly, the GRADE system was used to classify the strength of recommendations into four levels: strong recommendation, weak recommendation, recommendation to use interventions only in research, or no recommendation (32, 33). Two reviewers (KX and ZW) independently assessed the studies on the GRADE system during the evaluation process. Any disagreements were resolved by negotiation or with the participation of a third reviewer (XM or JW). Studies with no evidence for any outcome were excluded from the meta-analysis.
Outcomes
The outcomes were the evaluation of the effectiveness and safety of SARS-CoV-2 vaccines in older adults, which included vaccine effectiveness (VE), vaccine immunogenicity, and vaccine safety (VS). VE was defined as the percentage of participants infected by SARS-CoV-2 in relation to the total vaccinated population, with the infected group after vaccination including symptomatic individuals, laboratory-confirmed asymptomatic infections (infection after vaccination), individuals admitted to the hospital or ICU for COVID-19 (hospitalized or admitted to ICU after vaccination), and COVID-19-related deaths (death after vaccination). The immunogenicity of the vaccines was characterized by antibody seroconversion (AS) rate and geometric mean titer (GMT) of the relevant antibodies, which included neutralizing, anti-S (spike protein), and anti-RBD (spike protein receptor-binding domain) antibodies. VS was defined as the incidence of adverse events after the last vaccine dose had been administered, including total adverse events (AEs); solicited local adverse events (slAEs) such as pain, swelling, and redness; solicited systemic adverse events (ssAEs) such as fever, fatigue, and headache; and geriatric complications after vaccination.
Statistical analysis
Statistical analysis was performed using the Cochrane collaboration review management software (RevMan5.4). Binary variables representing the effectiveness and safety of the SARS-CoV-2 vaccines in comparison with a control group were expressed as odds ratios (ORs) and 95% confidence intervals (CIs), while continuous variables for the same measures in comparison with a control group were expressed in the form of standardized mean differences (SMDs) and 95% CIs. Heterogeneity was identified using the inconsistency (I2) metric. Degrees of statistical heterogeneity were considered to be low (I2 < 30%), moderate (I2 = 30%–50%), or high (I2 > 50%). The possible sources of heterogeneity were explored using sensitivity analysis. In cases where I2 was <50%, which represents low-to-moderate heterogeneity and no statistical heterogeneity among the studies, a fixed effects model was used. Otherwise, a random effects model was used for analysis (I2 ≥ 50%, which represents statistical heterogeneity among the studies). Publication bias was examined using Egger’s regression test and a funnel plot visual test; this was measured only when a subgroup contained three or more studies. Values of p < 0.05 were considered to represent statistical significance.
Results
Systematic literature search
The PRISMA literature retrieval flowchart is shown in Figure 1. A total of 1,260 potentially relevant articles were identified up to October 1, 2022, from electronic databases, including 306 from PubMed, 107 from Embase, 77 from the Cochrane Library, 100 from Web of Science, 13 from ClinicalTrials.gov, 657 from Research Square, and 0 from OpenGrey or other sources of gray literature. After preliminary screening, 110 duplicate records were excluded. After reading the titles and abstracts, 1,028 publications were then excluded in accordance with the inclusion and exclusion criteria. Subsequently, after reading the abstract and full text of each publication in detail, another 100 records were excluded due to insufficient data, unavailability of the full text, or no confirmed diagnosis. Ultimately, 22 studies were included in this meta-analysis based on the inclusion criteria.
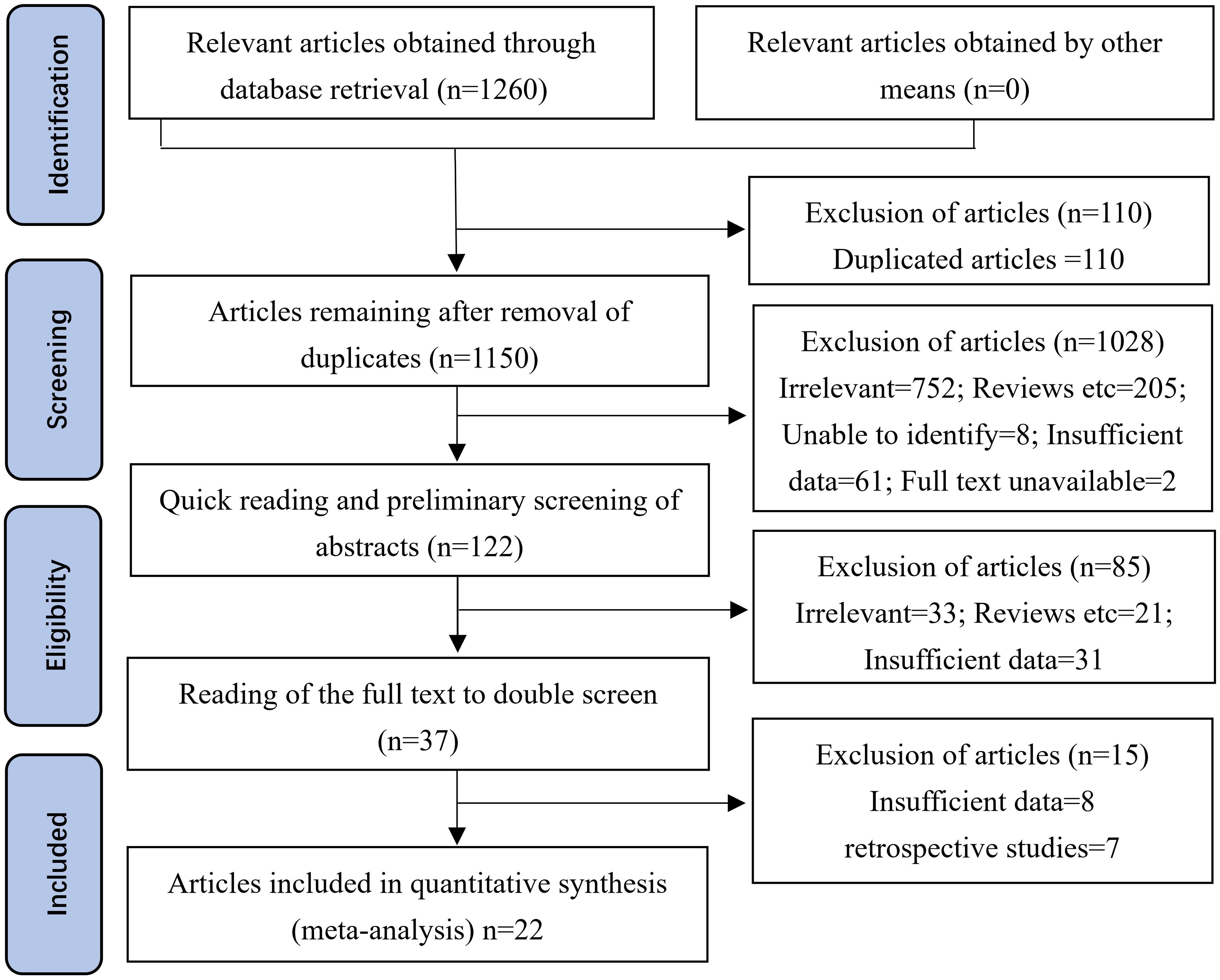
Figure 1 Methodological PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart for the selection of the studies included in this meta-analysis. A total of 306 studies were obtained from PubMed, 107 from Embase, 77 from the Cochrane Library, 100 from the Web of Science, 13 from ClinicalTrials.gov, 657 from Research Square, and 0 from OpenGrey or other sources of gray literature.
Basic characteristics
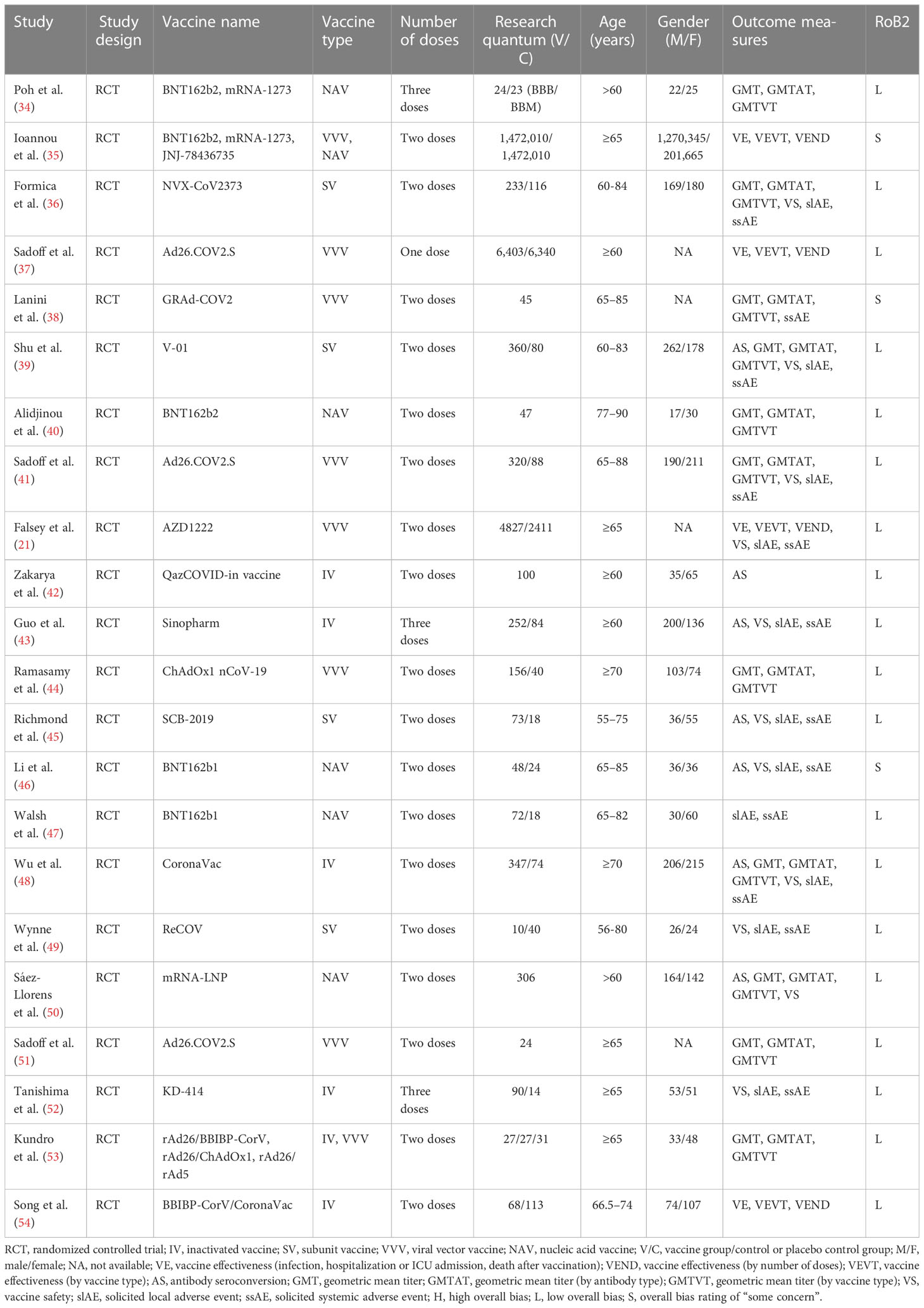
A total of 22 articles were included in the meta-analysis (21, 34–54), as shown in Table 1. From these publications, the relevant indicators were extracted, including information on the author, year of publication, number of participants, and vaccination efficacy and safety. Four articles reported data related to vaccination effectiveness (21, 35, 37, 54), 16 articles reported data related to antibody titer levels after vaccination (34, 36, 38–46, 48–51, 53), and 13 articles reported data related to the occurrence of vaccine-related adverse events in elderly people (21, 36, 38, 39, 41, 43, 45–49, 51, 52).
In addition to these, the same indicators (including author, year of publication, number of participants, and vaccine efficacy and safety) could be extracted from seven retrospective studies (55–61), shown in Appendix 5. Four articles reported on VE (58–61), while three articles reported antibody titer levels after vaccination (55–57) in the elderly. Six articles reported the effectiveness and safety of two vaccine doses (56–61), while only one article reported the effectiveness and safety of three vaccine doses (55) in elderly people. Five articles reported the effectiveness and safety of the nucleic acid vaccine (56, 58–61), while three articles reported the effectiveness and safety of the inactivated vaccine (55, 57, 58) in elderly people. Although vaccination was found to provide clear protection against SARS-CoV-2 infection in older adults, the results showed high and inexplicable heterogeneity in terms of both its effectiveness and its safety. Analyses of the data of these retrospective studies are presented in Appendix 5.
Finally, the same indicators (including author, year of publication, number of participants, and vaccination efficacy and safety) could be extracted from eight qualitative analysis articles (62–69), which are shown in Appendix 6. However, these studies could not be included in the meta-analysis due to insufficient data and/or descriptive explanations of vaccination efficacy and safety without the use of a parallel control, among other reasons. One article reported the VE (63), three articles reported antibody titer levels after vaccination (64, 65, 69), and six articles reported the occurrence of vaccine-related adverse events in the elderly (62, 64–68).
Quality assessment
The Cochrane Risk-of-Bias 2 tool (RoB 2 v9) was used to evaluate the quality of the individual studies included (Appendix 7). After evaluation, 19 articles were rated as low risk (21, 34, 36, 37, 39–45, 47–54), while the risk of bias for three articles was rated as “some concern” (35, 38, 46), as shown in Table 1 and Figure 2. Additionally, we used GRADEprofiler 3.6 to assess the quality of evidence for all systematic reviews. Of all the pieces of evidence included in the analysis, nine were characterized as high-quality evidence [VE, VEND (vaccine effectiveness by number of doses), VEVT (vaccine effectiveness by vaccine type), GMT, GMTAT (geometric mean titers by antibody type), GMTVT (geometric mean titers by vaccine type), AE, slAE, and ssAE], while one (AS) was characterized as evidence of moderate quality (Appendix 8). According to the quality evaluation using GRADE of the evidence on outcomes in terms of VE, immunogenicity, and VS, COVID-19 vaccination in older adults should be considered to be a strongly recommended strategy for control of COVID-19 through prevention of SARS-CoV-2 infection and reduction of COVID-19-related deaths.
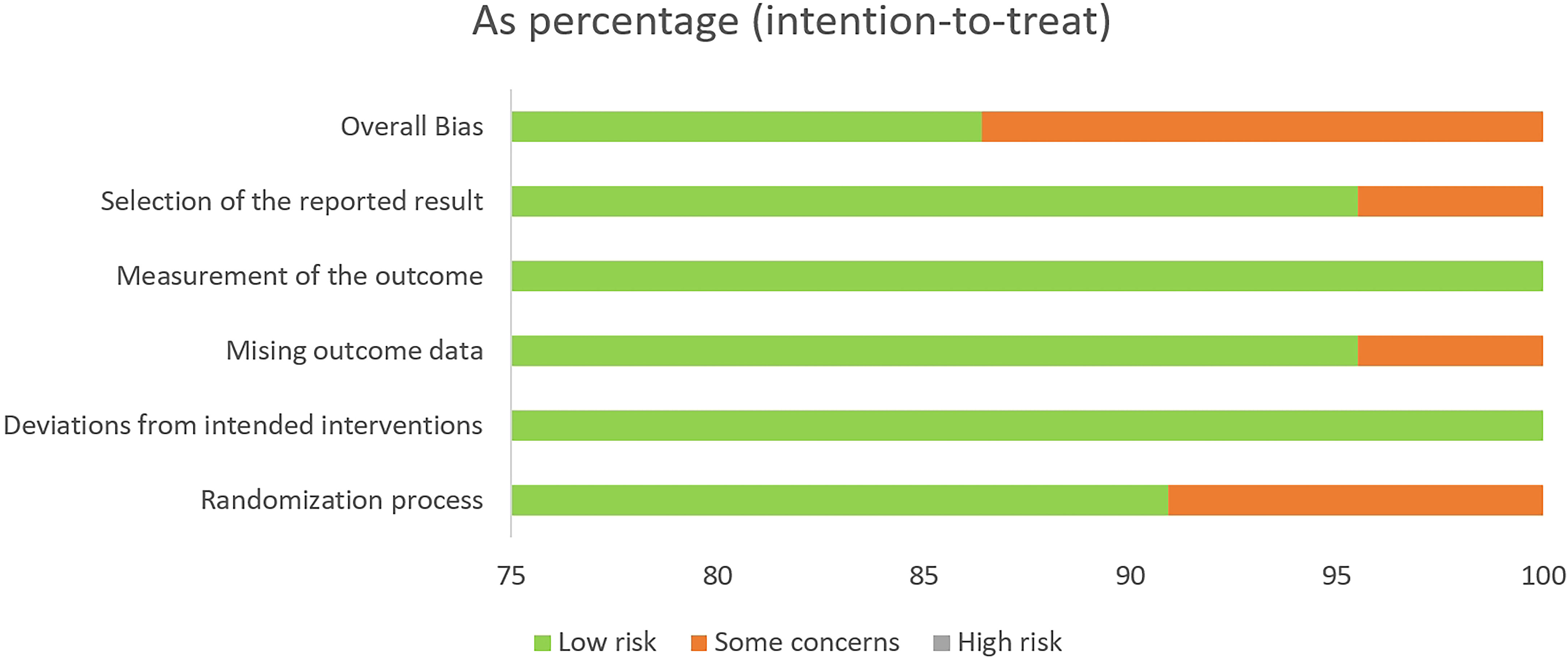
Figure 2 Risk of bias in the randomized controlled trials (RCTs) included, assessed using the Cochrane RoB2 tool.
Heterogeneity and risk of bias
Prior to meta-analysis of the included articles, a heterogeneity test was performed for results in which there were two or more included papers. The results of the analysis showed that no study significantly interfered with the results of the meta-analysis. The risk of publication bias was evaluated through funnel plots produced using Revman5.3; evidence of significant publication bias can be ignored due to the good levels of symmetry observed in these funnel plots. The shapes of the funnel plots for VE, AS, GMT, and VS are shown in Figure 3, while those of the subgroups are shown in Appendix 9. Groups with heterogeneity scores over 50 (I2 > 50%) were examined using Egger’s test; the results showed no evidence of publication bias (p > 0.05), except in the cases of GMT, anti-S of GMTAT, and nucleic acid vaccine of GMTVT groups (p < 0.05). Data from Egger’s test are shown in Appendix 10.
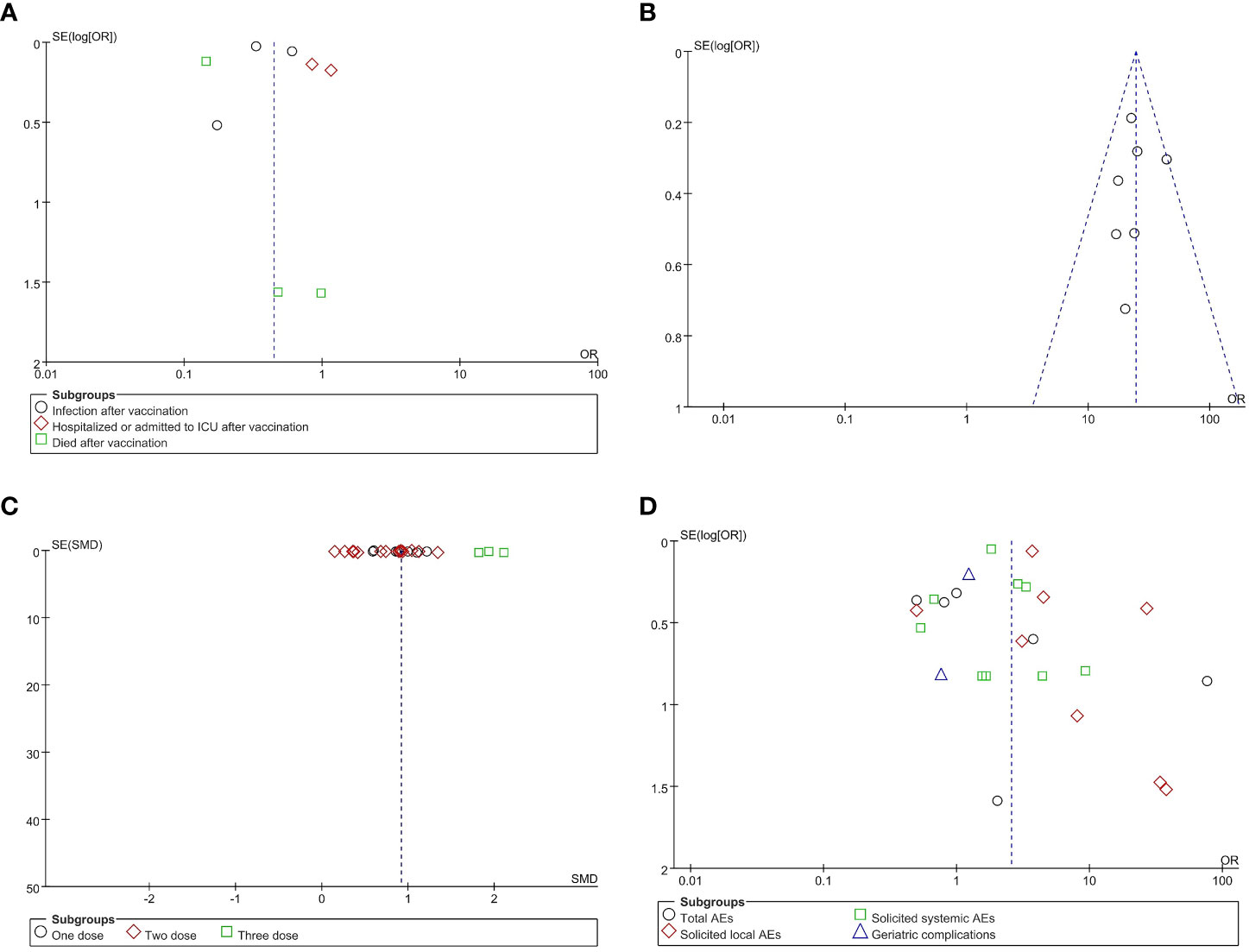
Figure 3 Funnel plots for publication bias. Publication bias in reports of vaccine effectiveness (VE) (A), antibody seroconversion (AS) (B), geometric mean titer (GMT) (C), and vaccine safety (VS) (D). OR, odds ratio; SMD, standardized mean difference.
Effectiveness of COVID-19 vaccines among older adults
Vaccine effectiveness
Four included studies contained data related to VE; these included a total of 1,711,591 and 1,709,676 participants in the vaccine and control groups, respectively. A random effects model was used for the meta-analysis due to the high heterogeneity (p < 0.00001, I2 = 95.4%) of the data (Figure 4). The meta-analysis on the effectiveness of the vaccine in this group of studies indicated an OR representing lower risk in the vaccine group compared to the control group (OR = 0.45, 95% CI = 0.28–0.70, p = 0.0005). The COVID-19 vaccines were shown to be more effective in preventing SARS-CoV-2 infection (OR = 0.38, 95% CI = 0.23–0.65, p = 0.0004) and in reducing COVID-19-related deaths (OR = 0.16; 95% CI = 0.10–0.25, p < 0.00001), but less effective in preventing hospitalization and ICU treatment (OR = 0.97, 95% CI = 0.71–1.33, p = 0.85) in elderly people. The subgroup analysis for each effectiveness indicator is shown in Appendix 11.
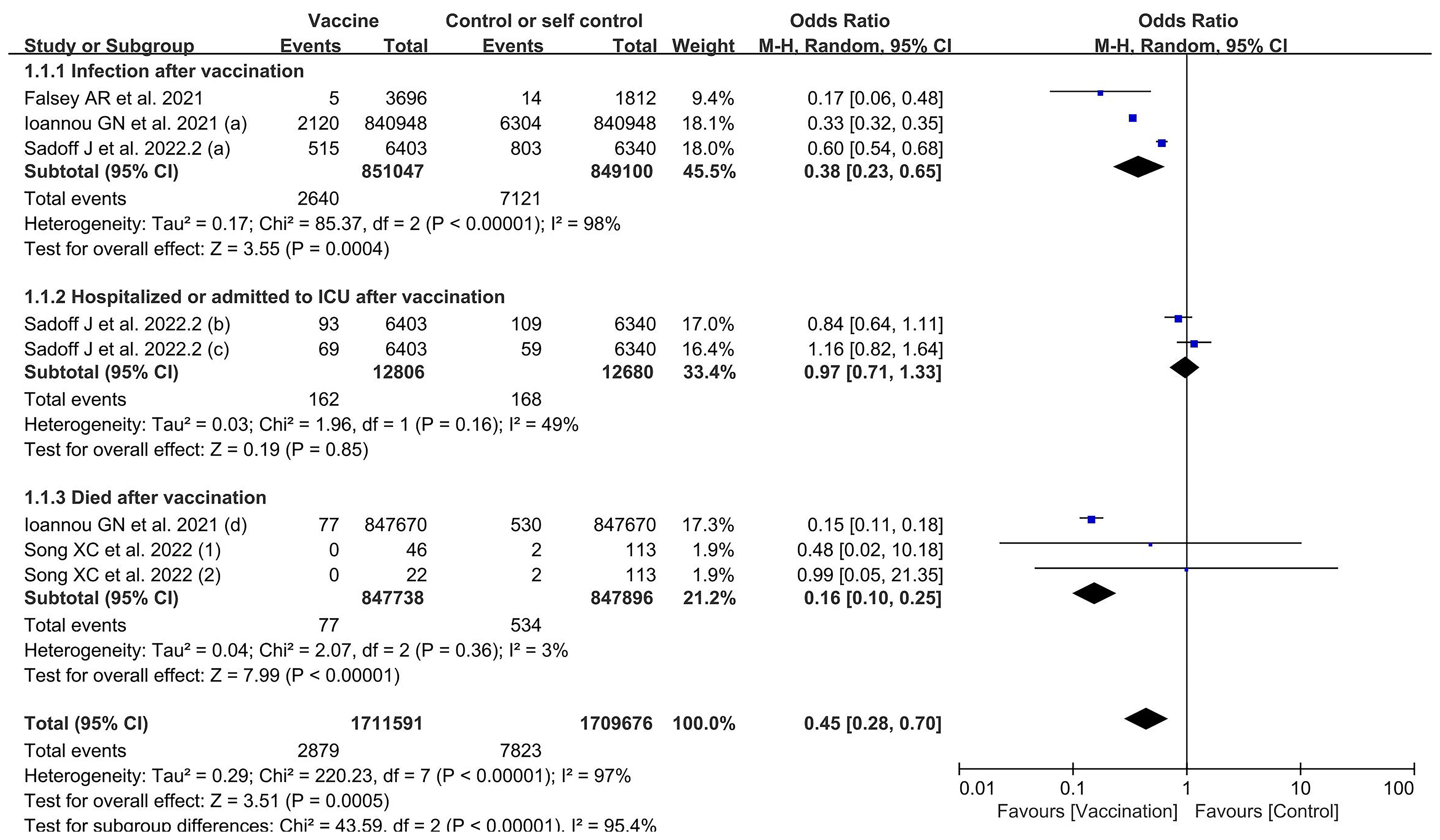
Figure 4 Forest plots of vaccine effectiveness by number of doses for the coronavirus disease 2019 (COVID-19) vaccine group compared with the control group. (a), infection after vaccination; (b), hospitalized after vaccination; (c), admitted to ICU after vaccination; (d), death after vaccination; 1, one dose; 2, two doses.
Subgroup analysis for vaccine effectiveness
The subgroup analysis for number of doses identified significant differences among four studies in the effects observed in experiments using one and two doses, which revealed that two vaccination doses had better effectiveness against SARS-CoV-2 infection compared to only one vaccination dose (χ2 = 10.24, p = 0.001, I2 = 90.2%) (Table 2 and Appendix 11). The outcomes demonstrated that the vaccinated group experienced better outcomes than the control group in both one-dose (OR = 0.81, 95% CI = 0.56–1.17, p = 0.26) and two-dose experiments (OR = 0.23, 95% CI = 0.11–0.45, p < 0.0001).
The subgroup analysis for vaccine type identified significant differences among four studies in the effects observed in experiments on the inactivated, nucleic acid, and viral vector vaccine groups (Table 2 and Appendix 11). The subgroup of studies with the nucleic acid vaccine showed better effectiveness compared to studies with the viral vector and inactivated vaccines (χ2 = 5.90, p = 0.05, I2 = 66.1%). The outcomes demonstrated that the vaccinated group experienced better outcomes than the control group in the experiments with inactivated (OR = 0.69, 95% CI = 0.08–6.00, p = 0.74), nucleic acid (OR = 0.22, 95% CI = 0.10–0.50, p = 0.0003), and viral vector vaccines (OR = 0.69, 95% CI = 0.46–1.05, p = 0.08).
Immunogenicity of COVID-19 vaccines among older adults
Antibody seroconversion rate
Seven included studies presented data related to AS rate; these included 1,584 participants in vaccine groups (Figure 5). A fixed effects model was used for the meta-analysis due to the low heterogeneity (p = 0.51, I2 = 0) of the data. The meta-analysis in the vaccine group found an OR indicating higher AS rates in the vaccinated groups compared with the control groups (OR = 24.42, 95%CI = 19.29–30.92, p < 0.00001).
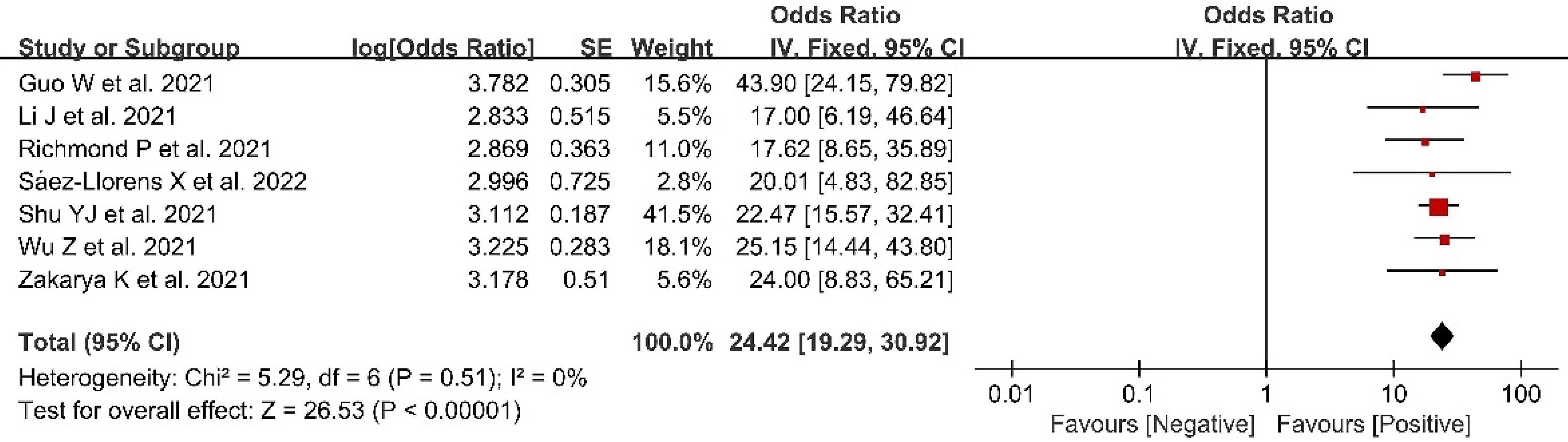
Figure 5 Forest plots of antibody seroconversion rate after coronavirus disease 2019 (COVID-19) vaccination.
Geometric mean titer
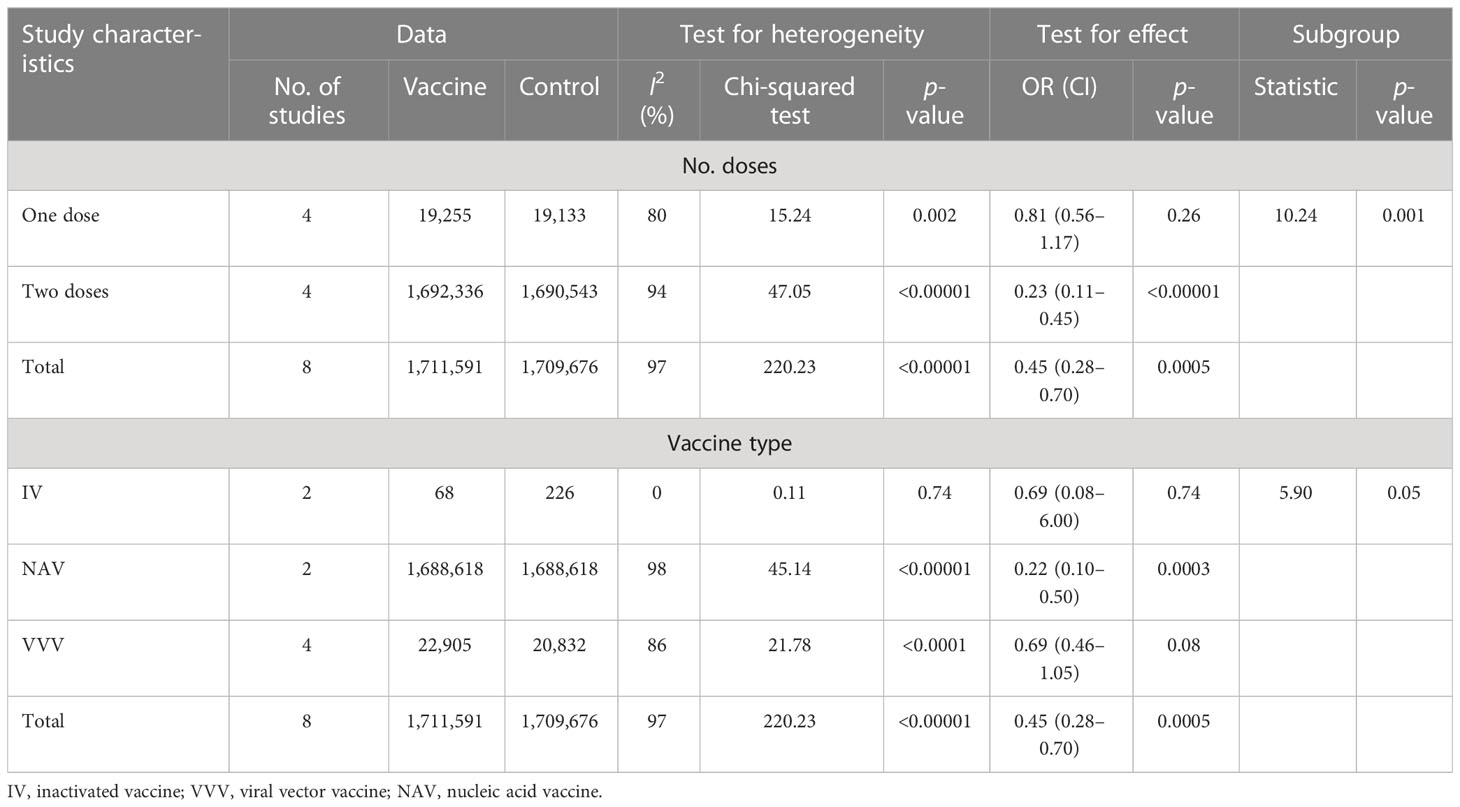
There were 11 included studies with data related to GMT; these included 2,312 and 1,072 participants in the vaccine and control groups, respectively (Figure 6). A random effects model was used for the meta-analysis due to the significant level of statistical heterogeneity (p < 0.00001, I2 = 91% > 50%) among studies. The pooled effects of these studies (SMD = 0.92, 95% CI = 0.64–1.20, Z = 6.41, p < 0.00001) showed that antibody titer levels improved significantly in the vaccine group, with a large effect compared to the control group. In addition, the subgroup analysis for number of doses found significant differences among 11 studies in the effects of experiments in which one dose, two doses, and three doses were administered (χ2= 2.09, p = 0.35, I2 = 4.3%). The three-dose subgroup showed better effectiveness than both the one-dose and two-dose subgroups. The outcomes demonstrated that the vaccine group experienced better outcomes than the control group in the experiments involving one dose (SMD = 0.84, 95% CI = 0.66–1.02, Z = 8.99, p < 0.00001), two doses (SMD = 0.73, 95% CI = 0.56–0.90, Z = 8.42, p < 0.00001), and three doses (SMD = 2.95, 95% CI = −0.65 to 6.55, Z = 1.61, p = 0.11).
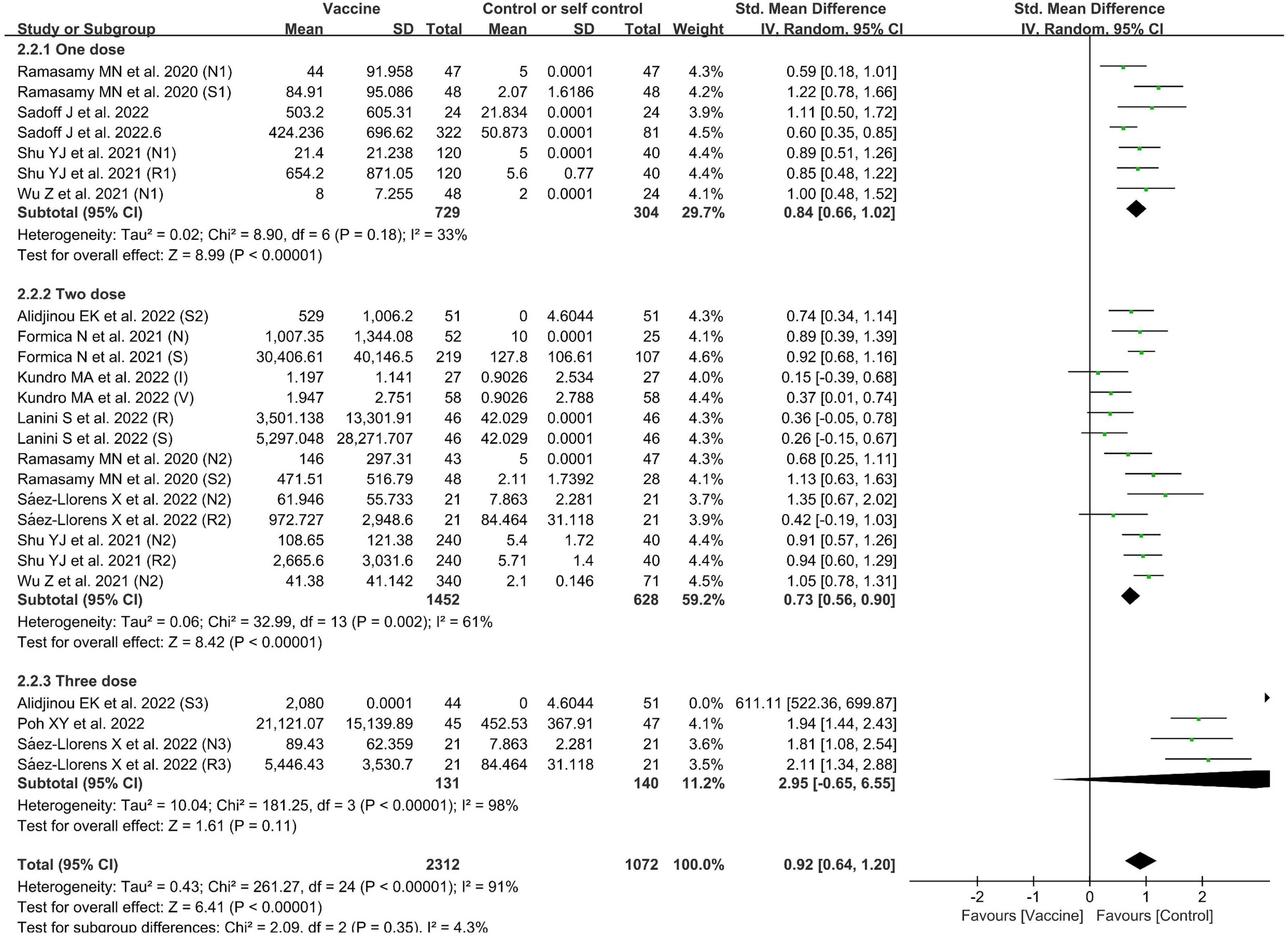
Figure 6 Forest plots of geometric mean titers (GMT) by number of doses for the coronavirus disease 2019 (COVID-19) vaccine group compared with the control group. N, neutralizing antibody; S, anti-S antibody; R, anti-RBD antibody; 1, one dose; 2, two doses; 3, three doses; I, inactivated vaccine; V, viral vector vaccine.
Subgroup analysis for GMT
The subgroup analysis of GMT for different antibody types found no statistical differences among the subgroups of neutralizing, anti-S, and anti-RBD antibodies (χ2= 0.32, p = 0.85, I2 = 0) (Table 3 and Appendix 11). The outcomes demonstrated that the vaccine group experienced better outcomes than the control group in results pertaining to neutralizing antibodies (SMD = 0.82, 95% CI = 0.64–1.01, Z = 8.73, p < 0.00001), anti-S antibodies (SMD = 1.11, 95% CI = 0.08–2.15, Z = 2.10, p = 0.004), and anti-RBD antibodies (SMD = 0.88, 95% CI = 0.44–1.31, Z = 3.94, p < 0.0001).
Although the nucleic acid vaccine showed better effectiveness compared to the inactivated, subunit, and viral vector vaccines (Table 3 and Appendix 11), the subgroup analysis for vaccine type found no statistical differences among 11 studies in the effects of experiments involving subunit, nucleic acid, and viral vector vaccine subgroups (χ2= 4.28, p = 0.23, I2 = 29.9%). The outcomes demonstrated that the vaccine group experienced better outcomes than the control group in the case of experiments involving inactivated vaccines (SMD = 0.76, 95% CI = 0.23–1.29, Z = 2.82, p = 0.005), subunit vaccines (SMD = 0.91, 95% CI = 0.77–1.04, Z = 12.88, p < 0.00001), nucleic acid vaccines (SMD = 1.57, 95% CI = 0.04–3.11, Z = 2.01, p = 0.004), and viral vector vaccines (SMD = 0.67, 95% CI = 0.46–0.88, Z = 6.13, p < 0.00001).
Safety of COVID-19 vaccines among older adults
Vaccine safety
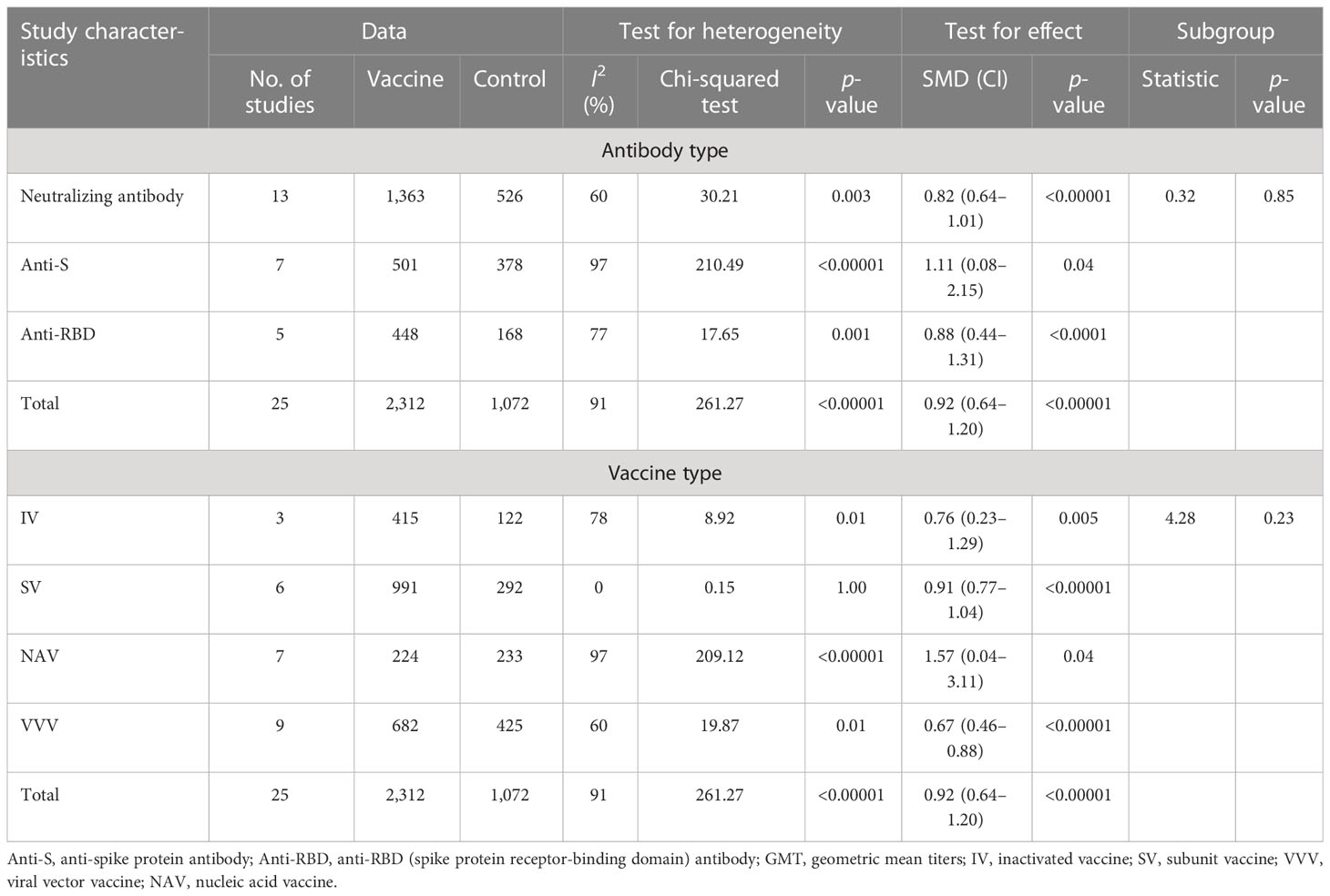
There were 10 included studies with data related to vaccine-related adverse events; these included 14,297 and 6,290 participants in the vaccine and control groups, respectively (Figure 7). A random effects model was used for the meta-analysis due to the high heterogeneity (p < 0.00001, I2 = 89%) of the data. The meta-analysis found an OR reflecting higher odds of adverse events in the vaccine group compared to the control group (OR = 2.57, 95% CI = 1.83–3.62, p < 0.00001). In addition, the subgroup analysis for immune effect found significant differences among 10 studies in the effects of experiments examining total AEs, slAEs, ssAEs, and geriatric complications after vaccination (χ2= 14.22, p = 0.003, I2 = 78.9%) (Figure 7). The outcomes demonstrated that the vaccine group experienced more AEs than the control group in the experiments on AEs (OR = 3.39, 95%CI = 1.01–11.40, p = 0.05), slAEs (OR = 6.45, 95%CI = 2.78–14.97, p < 0.0001), ssAEs (OR = 1.90, 95%CI = 1.24–2.92, p = 0.003), and geriatric complications (OR = 1.20; 95%CI = 0.82–1.76, p = 0.36).
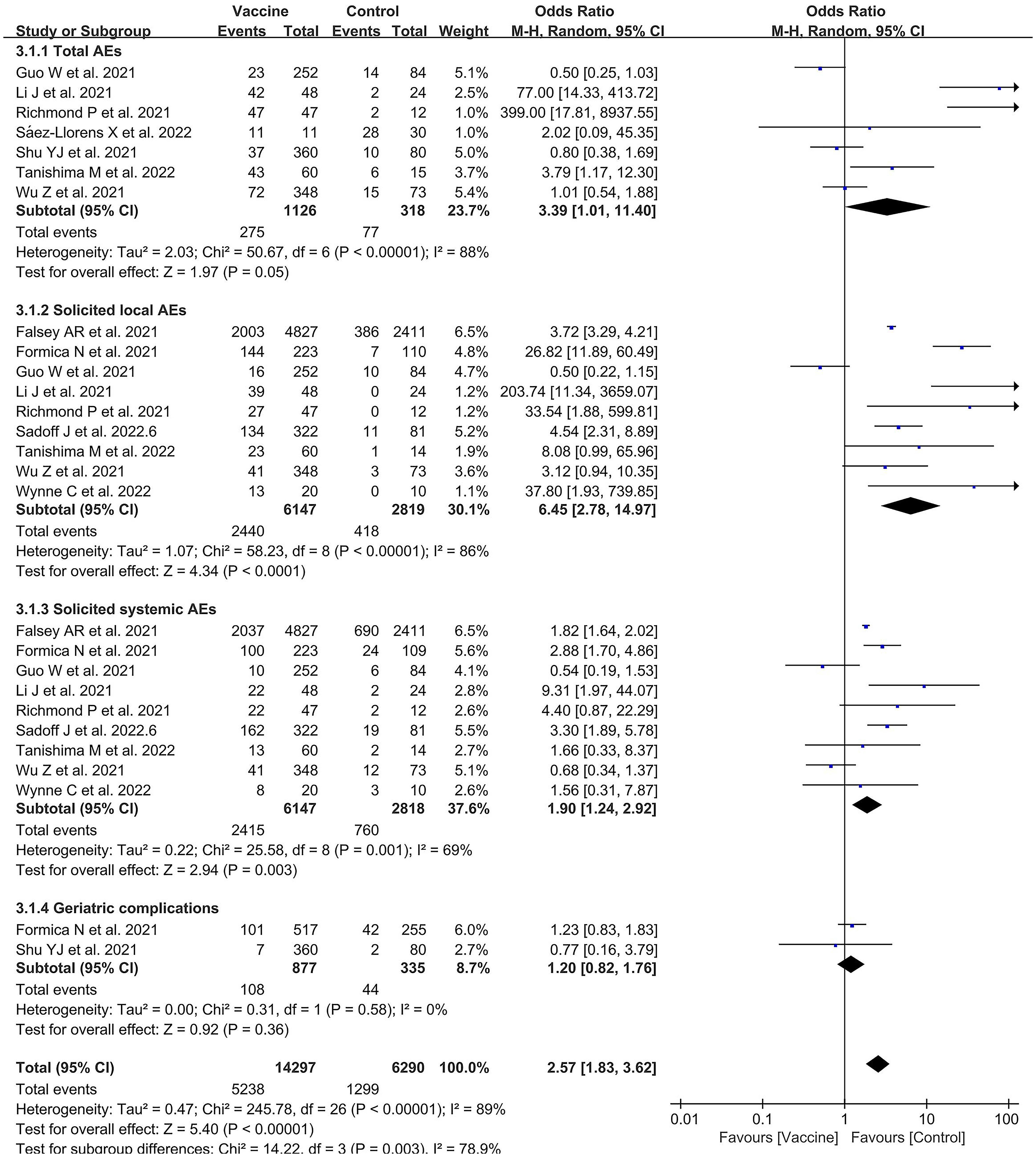
Figure 7 Forest plots of vaccine safety for the coronavirus disease 2019 (COVID-19) vaccine group compared with the control group.
Subgroup analysis for vaccine safety
A total of 10 studies included data related to the VS in terms of slAEs; these included 14,127 and 6,168 participants in the vaccine and control groups, respectively (Table 4 and Appendix 11). The random effects model was used for the meta-analysis due to the higher heterogeneity (p < 0.00001, I2 = 73%) of the data. The meta-analysis found an OR reflecting higher odds of slAEs in the vaccine group compared to the control group (OR = 3.82, 95% CI = 2.19–6.65, p < 0.00001). In addition, the subgroup analysis for immune effect found no statistical differences among the 10 studies in incidence of pain (OR = 5.04, 95% CI = 2.15–11.83, p = 0.0002), swelling (OR = 3.31, 95% CI = 0.89–12.28, p = 0.07), or redness (OR = 3.13, 95% CI = 0.90–10.94, p =0.07), χ2= 0.51, p = 0.78, I2 = 0.
There were 12 included studies with data related to the VS in terms of ssAEs; these included 19,545 and 8,639 participants in the vaccine and control groups, respectively (Table 4 and Appendix 11). A fixed effects model was used for the meta-analysis due to the lower heterogeneity (p = 0.05, I2 = 31%) of the data. The meta-analysis found a higher OR in the vaccine group compared to the control group (OR = 1.91, 95%CI = 1.75–2.09, p < 0.00001). In addition, the subgroup analysis for immune effect found differences among the 10 studies in terms of the effects on fever (OR = 5.38, 95% CI = 2.79–10.37, p < 0.00001), fatigue (OR = 1.65, 95% CI = 1.46–1.86, p < 0.00001), and headache (OR = 2.12, 95% CI = 1.85–2.44, p < 0.00001), χ2= 17.45, p = 0.0002, I2 = 88.5%.
Discussion
In this study, we assessed the effectiveness of COVID-19 vaccines against COVID-19 infection and their safety among older people. In the analysis of VE, we found that any dose of the vaccine is protective in elderly people; however, administration of two doses is more effective than one dose. The nucleic acid vaccines are more effective than other types, inactivated and viral vector vaccines, with the inactivated vaccine being the least effective of these three types. These three types of COVID-19 vaccine were more effective in preventing SARS-CoV-2 infection and in reducing deaths after infection, but less effective in preventing hospitalization and ICU treatment. It was found that elderly people who received COVID-19 vaccines experienced better outcomes (or the same level of outcome) than those who did not receive a COVID-19 vaccine in all aspects. It can be considered that vaccination for COVID-19 is still the major strategy for the prevention of SARS-CoV-2 infection and reduction of the severity of illness after infection. In terms of immunogenicity, the meta-analysis revealed high AS rates [including those of neutralizing antibodies, spike-specific immunoglobulin G (IgG), and RBD-specific IgG] in the elderly population after vaccination. Levels of all three of these antibody types, i.e., neutralizing, anti-S, and anti-RBD antibodies, increased significantly after vaccination, but there was no significant difference between them. Any number of vaccine doses was able to induce the production of antibodies in elderly people; however, the more frequent the inoculation doses, the higher the antibody titer levels were. There was not much difference in antibody titer levels between one or two doses, but the antibody titer levels increased significantly after three doses. With an increase in number of inoculation doses, the immune effect also increased correspondingly (65). Booster doses helped to increase the antibody titers and keep them stable. The standard fortification agent induces production of more antibody titers compared to the half-dose fortification agent and is more resistant to the delta variant than to the omicron variant (63).
Most of the approved COVID-19 vaccines, including the mRNA, recombinant adenovirus vector, inactivated coronavirus, and subunit vaccines, have been designed to elicit humoral and T-cell-mediated immune responses (70). In particular, COVID-19 vaccines can induce the production of anti-S, anti-RBD, and neutralizing antibodies against the spike protein of SARS-CoV-2 (71, 72), all of which bind to this spike protein and hinder its interaction with the angiotensin-converting enzyme 2 (ACE2) receptor (73). The viral entry of SARS-CoV-2 is facilitated by the interaction between its spike protein and the ACE2 receptor of the human host (74). Host protective antibodies induced by the COVID-19 vaccines hamper viral entry, the viral life cycle, and the pathogenicity of SARS-CoV-2. Although these antibody responses can be substantially boosted by two or three doses of the vaccines, the particular span of the duration of antibody responses remains unknown (75). The relevant findings of this meta-analysis once more suggests, in accordance with other reports on RCTs, that a second or booster dose of a vaccine triggers a considerable elevation in B-cell immune responses (57, 76). For this reason, the WHO recommends booster doses for priority vaccination groups, including elderly people, health workers, and other special groups (77, 78).
In this meta-analysis, it was found that, compared to the other types of vaccines, the nucleic acid vaccine produced the highest antibody titers in elderly people. This vaccine type induced the highest titer levels of spike-specific IgG, while the inactivated vaccine induced the lowest titer levels. Although the antibody titer levels of the elderly population after inoculation were lower than those of the younger population, the geometric mean ratios for antibodies were higher among the elderly population than among young people, which may have been a result of an insufficient number of studies involving the elderly population (64). In accordance with other reports, administration of the mRNA vaccine was associated with a significant increase in titer levels of neutralizing antibodies and in the antigen-specific production of IFN-γ, CD4+, and CD8+ T cells after the second vaccine dose (71, 72, 79). However, since only three articles involving this form of analysis were included, this conclusion may not be generalizable and may be limited to the vaccines included in the study. The analysis conducted in this study undoubtedly confirmed that the nucleic acid vaccine produced the best immune effect in the elderly population.
In the qualitative analysis, number of IFN-γ spot-forming cells (SFCs) and percentage of T-cell subsets were found to increase along with number of COVID-19 vaccine doses and time since inoculation among vaccinated elderly people (38, 44). The number of IFN-γ SFCs was slightly elevated on day 28 after the first dose of the viral vector vaccine compared to non-vaccination, but increased significantly after the second dose (44). After vaccination, the number of Th1 cells in the elderly population increased exponentially, while the number of Th2 cells fluctuated slightly with the increase in days since inoculation and number of doses (38, 66). Generally, the IFN response produced by alveolar macrophages, dendritic cells, natural killer cells, and inflammatory monocyte/macrophages is the primary antiviral innate immune signaling pathway (80). However, the number of articles reporting on RCTs examining T-cell responses and cytokine production after COVID-19 vaccination was insufficient, and these measures were not included in the analysis of protective immunity in the current study. Additionally, a number of older adults with diabetes or high blood pressure showed little difference in titer levels of neutralizing antibodies after vaccination when compared with healthy adults (69). Overall, the outcomes analysis of the retrospective studies showed that vaccination was effective in providing protection from SARS-CoV-2 infection, as well as in increasing the antibody positive conversion rate after vaccination.
In terms of the VS analysis, we found that vaccination will, to some extent, cause certain adverse reactions. The incidence of lAEs was higher than that of sAEs, and vaccination was not statistically associated with complications of geriatric diseases. Pain and fever were the most common lAEs and sAEs. Comparison of the pain and fever responses showed that pain was more prevalent than fever in the inoculated population. The frequency of local and systemic adverse reactions was higher after the first dose than after the second dose. In the meta-analysis, local adverse reactions after vaccination were more prevalent than systemic adverse reactions. The main reason for this may be that different elderly individuals perceive adverse reactions differently, and some elderly people are more sensitive to perceptions of physical injury (63, 64). Therefore, the previous assumption that inoculated groups are more sensitive to perceptions of physical injury is clearer.
In addition, we also found that the incidence of serious adverse reactions was very low. Some of the studies also reported on cardiovascular and cerebrovascular diseases arising in elderly people after vaccination, including vascular embolism, arrhythmias, and nervous system bleeding (81). Although severe adverse events have been reported at a rate of around five cases per one million in all those administered vaccine doses, this is extremely rare and is a very low rate (81). The data in the articles that covered such adverse reactions showed that the nucleic acid vaccine, compared with viral vector vaccination, is less likely to trigger cardiovascular and cerebrovascular diseases (68). Such reports of adverse events due to administration of COVID-19 vaccines have created vaccine hesitancy among elderly populations in many parts of the world. Millions of doses of the COVID-19 vaccines have already been administered around the world, and the safety of the vaccines has been frequently stressed by the many health authorities monitoring VS (82). It needs to be made abundantly clear to the elderly that the advantages of vaccination, which is the best method of controlling COVID-19 by preventing severe illness and related deaths, far outweigh any potential risks.
The present meta-analysis and systematic review have several limitations. First, this research was limited to studies published in Chinese and English, and there were some shortcomings in the inclusion of research published in other languages. Second, due to insufficient data, we were not able to conduct a subgroup analysis for comorbidities in the elderly population, such as diabetes, hypertension, and cancer. Third, in the study of outcome indicators, due to a lack of or inadequacy of relevant data, some studies were not comprehensive. For example, the analysis of vaccine effectiveness was not comprehensive enough due to insufficient data on elderly people after vaccination. Fourth, there was a large degree of heterogeneity among the included studies regarding VE, GMT, and VS. The results of the subgroup analyses should be interpreted with caution due to the diversity of influencing factors. Finally, there were not enough data to analyze long-term adverse effects after vaccination, and only short-term adverse effects, including slAEs and ssAEs, were analyzed in the current study. Moreover, in cases where the author could not be contacted to obtain detailed data, we used image extraction methods for data presented in images. Although there was no qualitative impact on the outcome indices, there were still some limitations in terms of the fine-grained data. In addition, we did not find enough data for a subgroup analysis of all types of vaccines for the elderly population. Nevertheless, this research provides some degree of insight into the effectiveness and safety of vaccination in the elderly population.
Conclusions
This systematic review and meta-analysis have comprehensively synthesized the latest data on vaccine effectiveness, immunogenicity, and safety in older adults based on 22 RCTs. In the meta-analysis, we found that vaccination is more effective in preventing SARS-CoV-2 infection and in reducing the number of COVID-19-related deaths in elderly people. The effect of two doses s stronger than that of one dose. After vaccination, high AS rates are observed in the elderly population. With an increase in the number of inoculation doses received, antibody titer levels also increased among the older population, with the highest antibody titer levels in elderly people being induced by the nucleic acid vaccinein. Vaccination can produce certain adverse reactions in the elderly population, but their incidence is quite low. It needs to be made abundantly clear to elderly people that the advantages of vaccination, which is the best way to control COVID-19 by preventing severe illness and reducing related deaths, far outweigh any potential risks. However, more randomized clinical trials are needed to increase the certainty of the evidence and to draw more reliable conclusions.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
KX and ZW performed the literature search and data extraction, and drafted the manuscript. MQ and YG were responsible for the quality assessment. NL, WX, and YZ conducted the statistical analysis. JW performed the literature search and data extraction. XM was responsible for the design and conceived the original idea. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project for Excellent Talents in Xihua University (grant no. 202026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1113156/full#supplementary-material
References
1. Wang C, Tee M, Roy AE, Fardin MA, Srichokchatchawan W, Habib HA, et al. The impact of COVID-19 pandemic on physical and mental health of asians: A study of seven middle-income countries in Asia. PLoS One (2021) 16:e0246824. doi: 10.1371/journal.pone.0246824
2. WHO coronavirus (COVID-19) dashboard. world health organization (2022). Available at: https://covid19.who.int/table (Accessed 1 October 2022).
3. Bonanad C, García-Blas S, Tarazona-Santabalbina F, Sanchis J, Bertomeu-González V, Fácila L, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc (2020) 21:915–8. doi: 10.1016/j.jamda.2020.05.045
4. Goldberg EM, Southerland LT, Meltzer AC, Pagenhardt J, Hoopes R, Camargo CA Jr, et al. Age-related differences in symptoms in older emergency department patients with COVID-19: Prevalence and outcomes in a multicenter cohort. J Am Geriatr Soc (2022) 70:1918–30. doi: 10.1111/jgs.17816
5. Vegivinti CTR, Evanson KW, Lyons H, Akosman I, Barrett A, Hardy N, et al. Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials. BMC Infect Dis (2022) 22:107. doi: 10.1186/s12879-022-07068-0
6. Nanda A, Vura NVRK, Gravenstein S. COVID-19 in older adults. Aging Clin Exp Res (2020) 32:1199–202. doi: 10.1007/s40520-020-01581-5
7. Daoust JF. Elderly people and responses to COVID-19 in 27 countries. PLoS One (2020) 15:e0235590. doi: 10.1371/journal.pone.0235590
8. Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, et al. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res Rev (2021) 65:101205. doi: 10.1016/j.arr.2020.101205
9. Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing (2021) 50:279–83. doi: 10.1093/ageing/afaa274
10. Dhama K, Patel SK, Natesan S, Vora KS, Iqbal Yatoo M, Tiwari R, et al. COVID-19 in the elderly people and advances in vaccination approaches. Hum Vaccin Immunother (2020) 16:2938–43. doi: 10.1080/21645515.2020.1842683
11. Witham MD, Gordon AL, Henderson EJ, Harwood RH. Pandemic research for older people: doing it better next time. Age Ageing (2021) 50:276–8. doi: 10.1093/ageing/afaa273
12. COVID-19 vaccine tracker and landscape. world health organization (2022). Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed 1 October 2022).
13. COVID-19 vaccines WHO emergency use listing (EUL) issued. world health organization (2022). Available at: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (Accessed 1 October 2022).
14. The status of COVID-19 vaccines within WHO EUL/PQ evaluation process. world health organization (2022). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (Accessed 1 October 2022).
15. Ouyang H, Ma X, Wu X. The prevalence and determinants of COVID-19 vaccine hesitancy in the age of infodemic. Hum Vaccin Immunother (2022) 18:2013694. doi: 10.1080/21645515.2021.2013694
16. Shao Y, Wu Y, Feng Y, Xu W, Xiong F, Zhang X. SARS-CoV-2 vaccine research and immunization strategies for improved control of the COVID-19 pandemic. Front Med (2022) 16:185–95. doi: 10.1007/s11684-021-0913-y
17. Adhikari B, Cheah PY. Vaccine hesitancy in the COVID-19 era. Lancet Infect Dis (2021) 21:1086. doi: 10.1016/S1473-3099(21)00390-X
18. World Health Organization. Ten threats to global health in 2019. world health organization (2021). Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (Accessed 1 October 2022).
19. Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med (2021) 385:1761–73. doi: 10.1056/NEJMoa2110345
20. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
21. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) covid-19 vaccine. N Engl J Med (2021) 385:2348–60. doi: 10.1056/NEJMoa2105290
22. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med (2021) 384:2187–201. doi: 10.1056/NEJMoa2101544
23. Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet (2021) 398:2173–84. doi: 10.1016/S0140-6736(21)02000-6
24. Wanlapakorn N, Suntronwong N, Phowatthanasathian H, Yorsaeng R, Vichaiwattana P, Thongmee T, et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: A prospective cohort study. Hum Vaccin Immunother (2022) 18:2029111. doi: 10.1080/21645515.2022.2029111
25. Publications of COVID-19 vaccine. world health organization (2022). Available at: https://www.who.int/publications/i (Accessed 1 October 2022).
26. Cox LS, Bellantuono I, Lord JM, Sapey E, Mannick JB, Partridge L, et al. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev (2020) 1:e55–7. doi: 10.1016/S2666-7568(20)30011-8
27. Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother (2013) 9:1364–7. doi: 10.4161/hv.24696
28. Ben-David BM, Keisari S, Palgi Y. Vaccine and psychological booster: factors associated with older adults' compliance to the booster COVID-19 vaccine in Israel. J Appl Gerontol (2022) 41:1636–40. doi: 10.1177/07334648221081982
29. Spetz M, Lundberg L, Nwaru C, Li H, Santosa A, Leach S, et al. The social patterning of covid-19 vaccine uptake in older adults: a register-based cross-sectional study in Sweden. Lancet Reg Health Eur (2022) 15:100331. doi: 10.1016/j.lanepe.2022.100331
30. Bhagianadh D, Arora K. COVID-19 vaccine hesitancy among community-dwelling older adults: The role of information sources. J Appl Gerontol (2022) 41:4–11. doi: 10.1177/07334648211037507
31. The risk of bias 2 (RoB 2) tool is an update to the original risk of bias tool that launched in 2008. cochrane library of cochrane methods (2022). Available at: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (Accessed 1 February 2022).
32. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
33. Jorda A, Kussmann M, Kolenchery N, Siller-Matula JM, Zeitlinger M, Jilma B, et al. Convalescent plasma treatment in patients with covid-19: A systematic review and meta-analysis. Front Immunol (2022) 13:817829. doi: 10.3389/fimmu.2022.817829
34. Poh XY, Tan CW, Lee IR, Chavatte JM, Fong SW, Prince T, et al. Antibody response of heterologous vs homologous mRNA vaccine boosters against the SARS-CoV-2 omicron variant: Interim results from the PRIBIVAC study, a randomized clinical trial. Clin Infect Dis (2022) 75(12):2088–96. doi: 10.1093/cid/ciac345
35. Ioannou GN, Locke ER, O'Hare AM, Bohnert ASB, Boyko EJ, Hynes DM, et al. COVID-19 vaccination effectiveness against infection or death in a national US health care system a target trial emulation study. Ann Of Internal Med (2021) 175:352. doi: 10.7326/M21-3256
36. Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: A phase 2 randomized placebo-controlled trial. PloS Med (2021) 18:e1003769. doi: 10.1371/journal.pmed.1003769
37. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med (2022) 386:847–60. doi: 10.1056/NEJMoa2117608
38. Lanini S, Capone S, Antinori A, Milleri S, Nicastri E, Camerini R, et al. GRAd-COV2, a gorilla adenovirus-based candidate vaccine against COVID-19, is safe and immunogenic in younger and older adults. Sci Transl Med (2022) 14:eabj1996. doi: 10.1126/scitranslmed.abj1996
39. Shu YJ, He JF, Pei RJ, He P, Huang ZH, Chen SM, et al. Immunogenicity and safety of a recombinant fusion protein vaccine (V-01) against coronavirus disease 2019 in healthy adults: a randomized, double-blind, placebo-controlled, phase II trial. Chin Med J (Engl) (2021) 134:1967–76. doi: 10.1097/CM9.0000000000001702
40. Alidjinou EK, Demaret J, Corroyer-Simovic B, Labreuche J, Goffard A, Trauet J, et al. Immunogenicity of BNT162b2 vaccine booster against SARS-CoV-2 delta and omicron variants in nursing home residents: a prospective observational study in older adults aged from 68 to 98 years. Lancet Reg Health Eur (2022) 17:100385. doi: 10.1016/j.lanepe.2022.100385
41. Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S covid-19 vaccine. N Engl J Med (2021) 384:1824–35. doi: 10.1056/NEJMoa2034201
42. Zakarya K, Kutumbetov L, Orynbayev M, Abduraimov Y, Sultankulova K, Kassenov M, et al. Safety and immunogenicity of a QazCovid-in® inactivated whole-virion vaccine against COVID-19 in healthy adults: A single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. E Clin Med (2021) 39:101078. doi: 10.1016/j.eclinm.2021.101078
43. Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: A randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine (2021) 38:101010. doi: 10.1016/j.eclinm.2021.101010
44. Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet (2021) 396:1979–93. doi: 10.1016/S0140-6736(20)32466-1
45. Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, et al. Safety and immunogenicity of s-trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet (2021) 397:682–94. doi: 10.1016/S0140-6736(21)00241-5
46. Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: A randomized, placebo-controlled, double-blind phase 1 study. Nat Med (2021) 27:1062–70. doi: 10.1038/s41591-021-01330-9
47. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med (2020) 383:2439–50. doi: 10.1056/NEJMoa2027906
48. Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis (2021) 21:803–12. doi: 10.1016/S1473-3099(20)30987-7
49. Wynne C, Hamilton P, Li J, Mo C, Yu J, Yao W, et al. The preliminary safety and immunogenicity results of a randomized, double-blind, placebo-controlled phase I trial for a recombinant two-component subunit SARS-CoV-2 vaccine ReCOV. medRxiv (2022). doi: 10.1101/2022.05.11.22274932
50. Sáez-Llorens X, Lanata C, Aranguren E, Celis CR, Cornejo R, DeAntonio R, et al. Safety and immunogenicity of mRNA-LNP COVID-19 vaccine CVnCoV in Latin American adults: A phase 2 randomized study. Vaccine X. (2022) 11:100189. doi: 10.1016/j.jvacx.2022.100189
51. Sadoff J, Le Gars M, Brandenburg B, Cárdenas V, Shukarev G, Vaissiere N, et al. Durable antibody responses elicited by 1 dose of Ad26.COV2.S and substantial increase after boosting: 2 randomized clinical trials. Vaccine (2022) 40:4403–11. doi: 10.1016/j.vaccine.2022.05.047
52. Tanishima M, Ibaraki K, Kido K, Nakayama S, Ata K, Nakamura H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, KD-414, in healthy adult and elderly subjects: A randomized, double-blind, placebo-controlled, phase 1/2 clinical study in Japan. medRxiv (2022). doi: 10.1101/2022.06.28.22276794
53. Kundro MA, Losso MH, Macchia A, Pastor I, Alonso Serena M, Gestoso C, et al. Safety and immunogenicity of heterologous COVID-19 vaccine regimens to deal with product shortage: A randomised clinical trial in an elderly population. Public Health Pract (Oxf) (2022) 4:100313. doi: 10.1016/j.puhip.2022.100313
54. Song XC, Zhou XH, Cheng JH, Zhang WH, Shen X, Xu H, et al. The roles of inactivated vaccines in older patients with infection of delta variant in nanjing, China. Aging (Albany NY) (2022) 14:4211–9. doi: 10.18632/aging.204085
55. Bag Soytas R, Cengiz M, Islamoglu MS, Borku Uysal B, Yavuzer S, Yavuzer H. Antibody responses to COVID-19 vaccines in older adults. J Med Virol (2022) 94:1650–4. doi: 10.1002/jmv.27531
56. San Román J, Candel FJ, Sanz JC, López P, Menéndez-Colino R, Barreiro P, et al. Humoral and cellular response after mRNA vaccination in nursing homes: Influence of age and of history of COVID-19. Vaccines (Basel) (2022) 10(3):383. doi: 10.3390/vaccines10030383
57. Schultz BM, Melo-González F, Duarte LF, Gálvez NMS, Pacheco GA, Soto JA, et al. A booster dose of CoronaVac increases neutralizing antibodies and T cells that recognize delta and omicron variants of concern. mBio (2022) 13:e0142322. doi: 10.1128/mbio.01423-22
58. Arregocés-Castillo L, Fernández-Niño J, Rojas-Botero M, Palacios-Clavijo A, Galvis-Pedraza M, Rincón-Medrano L, et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev (2022) 3:e242–52. doi: 10.1016/S2666-7568(22)00035-6
59. Meyer M, Constancias F, Worth C, Meyer A, Muller M, Boussuge A, et al. Humoral immune response after COVID-19 infection or BNT162b2 vaccine among older adults: Evolution over time and protective thresholds. Geroscience (2022) 44:1229–40. doi: 10.1007/s11357-022-00546-y
60. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data [published correction appears in lancet. Lancet (2021) 397:1819–29. doi: 10.1016/S0140-6736(21)00947-8
61. Nunes B, Rodrigues AP, Kislaya I, Cruz C, Peralta-Santos A, Lima J, et al. mRNA vaccine effectiveness against COVID-19-related hospitalisations and deaths in older adults: A cohort study based on data linkage of national health registries in Portugal, February to august 2021. Euro Surveill (2021) 26:2100833. doi: 10.2807/1560-7917.ES.2021.26.38.2100833
62. Jose M, Rajmohan P, Thomas J, Krishna S, Antony B, Gopinathan UU, et al. Active symptom-based surveillance of adverse events following immunization among individuals vaccinated with ChAdOx1 nCoV-19 coronavirus vaccine in a tertiary hospital of kerala. Curr Drug Saf (2022) 17:327–34. doi: 10.2174/1574886317666220207120649
63. Nantanee R, Jantarabenjakul W, Jaru-Ampornpan P, Sodsai P, Himananto O, Athipunjapong J, et al. A randomized clinical trial of a fractional low dose of BNT162b2 booster in adults following AZD1222. Vaccines (Basel) (2022) 10:914. doi: 10.3390/vaccines10060914
64. Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet (2022) 399:521–9. doi: 10.1016/S0140-6736(22)00094-0
65. Sridhar S, Joaquin A, Bonaparte MI, Bueso A, Chabanon AL, Chen A, et al. Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: Interim findings from a phase 2, randomised, dose-finding, multicentre study. Lancet Infect Dis (2022) 22:636–48. doi: 10.1016/S1473-3099(21)00764-7
66. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med (2020) 383:2427–38. doi: 10.1056/NEJMoa2028436
67. Choi YY, Kim MK, Kwon HC, Kim GH. Safety monitoring after the BNT162b2 COVID-19 vaccine among adults aged 75 years or older. J Korean Med Sci (2021) 36:e318. doi: 10.3346/jkms.2021.36.e318
68. Cari L, Alhosseini MN, Fiore P, Pierno S, Pacor S, Bergamo A, et al. Cardiovascular, neurological, and pulmonary events following vaccination with the BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines: An analysis of European data. J Autoimmun (2021) 125:102742. doi: 10.1016/j.jaut.2021.102742
69. Zhang Y, Chen H, Lv J, Huang T, Zhang R, Zhang D, et al. Evaluation of immunogenicity and safety of vero cell-derived inactivated COVID-19 vaccine in older patients with hypertension and diabetes mellitus. Vaccines (Basel) (2022) 10:1020. doi: 10.3390/vaccines10071020
70. Noor R. Host protective immunity against severe acute respiratory coronavirus 2 (SARS-CoV-2) and the COVID-19 vaccine-induced immunity against SARS-CoV-2 and its variants. Viruses (2022) 14(11):2541. doi: 10.3390/v14112541
71. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol (2021) 21:475–84. doi: 10.1038/s41577-021-00578-z
72. Jamshidi E, Asgary A, Shafiekhani P, Khajeamiri Y, Mohamed K, Esmaily H, et al. Longevity of immunity following COVID-19 vaccination: A comprehensive review of the currently approved vaccines. Hum Vaccines Immunother (2022) 18:2037384. doi: 10.1080/21645515.2022.2037384
73. Zhang Y, Zhang L, Wu J, Yu Y, Liu S, Li T, et al. A second functional furin site in the SARS-CoV-2 spike protein. Emerg Microbes Infect (2022) 11:182–94. doi: 10.1080/22221751.2021.2014284
74. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med (2020) 383:1920–31. doi: 10.1056/NEJMoa2022483
75. Zhang J, Xing S, Liang D, Hu W, Ke C, He J, et al. Differential antibody response to inactivated COVID-19 vaccines in healthy subjects. Front Cell Infect Microbiol (2021) 11:791660. doi: 10.3389/fcimb.2021.791660
76. Noor R. A review on the induction of host immunity by the current COVID-19 vaccines and a brief non-pharmaceutical intervention to mitigate the pandemic. Bull Natl Res Cent (2022) 46:31. doi: 10.1186/s42269-022-00719-x
77. Interim recommendations for use of the cansino Ad5-Ncov-S vaccine (Convidecia ®) against covid-19. world health organization (2022). Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad5-nCoV-Convidecia (Accessed 19 May 2022).
78. Updated who interim recommendations for the use of the janssen Ad26.Cov2.S (Covid-19) vaccine. world health organization (2022). Available at: https://www.who.int/news/item/06-06-2022-updated-who-interim-recommendations-for-the-use-of-thejanssen-ad26.cov2.s-(covid-19)-vaccine (Accessed 1 October 2022).
79. Zhu F, Jin P, Zhu T, Wang W, Ye H, Pan H, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored coronavirus disease 2019 (COVID-19) vaccine with a homologous prime-boost regimen in healthy participants aged ≥6 years: A randomized, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis (2022) 75(1):e783–91. doi: 10.1093/cid/ciab845
80. Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe (2021) 29:1052–62. doi: 10.1016/j.chom.2021.05.004
81. Centers for disease control and prevention selected adverse events reported after COVID-19 vaccination (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (Accessed 1 October 2022).
Keywords: COVID-19 vaccine, older adults, SARS-CoV-2, effectiveness, safety, meta-analysis
Citation: Xu K, Wang Z, Qin M, Gao Y, Luo N, Xie W, Zou Y, Wang J and Ma X (2023) A systematic review and meta-analysis of the effectiveness and safety of COVID-19 vaccination in older adults. Front. Immunol. 14:1113156. doi: 10.3389/fimmu.2023.1113156
Received: 01 December 2022; Accepted: 10 February 2023;
Published: 03 March 2023.
Edited by:
Ritthideach Yorsaeng, Chulalongkorn University, ThailandReviewed by:
Ana Katherine Gonçalves, Federal University of Rio Grande do Norte, BrazilRasha Ashmawy, Ministry of Health and Population, Egypt
Copyright © 2023 Xu, Wang, Qin, Gao, Luo, Xie, Zou, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingming Ma, ming2020xm@163.com
 Kun Xu
Kun Xu Zihan Wang
Zihan Wang Maorong Qin
Maorong Qin Yangyu Gao
Yangyu Gao Na Luo1
Na Luo1 Jie Wang
Jie Wang Xingming Ma
Xingming Ma